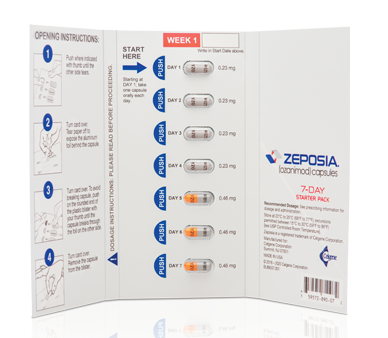
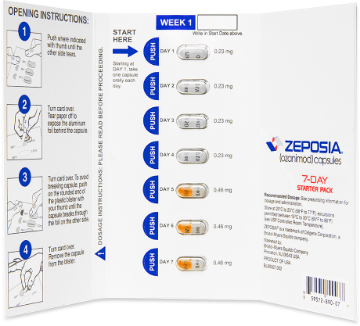
INDICATION
ZEPOSIA® (ozanimod) is indicated for the treatment of moderately to severely active ulcerative colitis (UC) in adults.
IMPORTANT SAFETY INFORMATION
Contraindications:
- Patients who in the last 6 months, experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure or have a presence of Mobitz type II second-degree or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial block, unless the patient has a functioning pacemaker
- Patients with severe untreated sleep apnea
- Patients taking a monoamine oxidase (MAO) inhibitor
Infections: ZEPOSIA may increase the susceptibility to infections. Life-threatening and rare fatal infections have occurred in patients receiving ZEPOSIA. Obtain a recent (i.e., within 6 months or after discontinuation of prior UC therapy) complete blood count (CBC) including lymphocyte count before initiation of ZEPOSIA. Delay initiation of ZEPOSIA in patients with an active infection until the infection is resolved. Consider interruption of treatment with ZEPOSIA if a patient develops a serious infection. Continue monitoring for infections up to 3 months after discontinuing ZEPOSIA.
- Herpes zoster and herpes simplex were seen in clinical trials of ZEPOSIA. Herpes simplex encephalitis and varicella zoster meningitis have been reported with sphingosine 1-phosphate (S1P) receptor modulators. Patients without a healthcare professional-confirmed history of varicella (chickenpox), or without documentation of a full course of vaccination against varicella zoster virus (VZV), should be tested for antibodies to VZV before initiating ZEPOSIA.
- Cases of fatal cryptococcal meningitis (CM) and disseminated cryptococcal infections have been reported with S1P receptor modulators. If CM is suspected, ZEPOSIA should be suspended until cryptococcal infection has been excluded. If CM is diagnosed, appropriate treatment should be initiated.
- In the UC clinical studies, patients who received ZEPOSIA were not to receive concomitant treatment with antineoplastic, non-corticosteroid immunosuppressive, or immune-modulating therapies used for treatment of UC. Concomitant use of ZEPOSIA with any of these therapies would be expected to increase the risk of immunosuppression. When switching to ZEPOSIA from immunosuppressive medications, consider the duration of their effects and their mode of action to avoid unintended additive immunosuppressive effects.
- Use of live attenuated vaccines should be avoided during and for 3 months after treatment with ZEPOSIA. If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of ZEPOSIA.
Progressive Multifocal Leukoencephalopathy (PML): PML is an opportunistic viral infection of the brain that typically occurs in patients who are immunocompromised, and that usually leads to death or severe disability.
PML has been reported in patients treated with S1P receptor modulators, including ZEPOSIA, and other UC therapies and has been associated with some risk factors (e.g., immunocompromised patients, polytherapy with immunosuppressants, duration of use). If PML is suspected, withhold ZEPOSIA and perform an appropriate diagnostic evaluation. If confirmed, treatment with ZEPOSIA should be discontinued.
Bradyarrhythmia and Atrioventricular Conduction Delays: Since initiation of ZEPOSIA may result in a transient decrease in heart rate and atrioventricular conduction delays, dose titration is recommended to help reduce cardiac effects. If treatment with ZEPOSIA is considered, advice from a cardiologist should be sought for those individuals:
- with significant QT prolongation
- with arrhythmias requiring treatment with Class 1a or III anti-arrhythmic drugs
- with ischemic heart disease, heart failure, history of cardiac arrest or myocardial infarction, cerebrovascular disease, and uncontrolled hypertension
- with a history of Mobitz type II second-degree or higher AV block, sick sinus syndrome, or sino-atrial heart block
Liver Injury: Clinically significant liver injury, including acute liver failure requiring transplant, has occurred in patients treated with ZEPOSIA in the postmarketing setting. Signs of liver injury, including elevated serum hepatic enzymes and elevated total bilirubin, have occurred as early as ten days after the first dose. Obtain transaminase and bilirubin levels, if not recently available (i.e., within 6 months), before initiation of ZEPOSIA. Obtain transaminase levels and total bilirubin levels periodically during treatment and until two months after ZEPOSIA discontinuation. Patients should be monitored for signs and symptoms of any hepatic injury. Patients who develop symptoms suggestive of hepatic dysfunction should have hepatic enzymes promptly checked, and ZEPOSIA should be interrupted. Treatment should not be resumed if a plausible alternative etiology for the signs and symptoms cannot be established, because these patients are at risk for severe drug-induced liver injury.
Fetal Risk: There are no adequate and well-controlled studies in pregnant women. Based on animal studies, ZEPOSIA may cause fetal harm. Women of childbearing potential should use effective contraception to avoid pregnancy during treatment and for 3 months after stopping ZEPOSIA.
Increased Blood Pressure: Increase in systolic pressure was observed after about 3 months of treatment and persisted throughout treatment. Blood pressure should be monitored during treatment and managed appropriately.
Respiratory Effects: ZEPOSIA may cause a decline in pulmonary function. Spirometric evaluation of respiratory function should be performed during therapy, if clinically indicated.
Macular Edema: S1P modulators have been associated with an increased risk of macular edema. Obtain a baseline evaluation of the fundus, including a macula, near the start of treatment with ZEPOSIA. Perform an examination of the fundus, including the macula, periodically while on therapy and any time there is a change in vision. Continuation of ZEPOSIA therapy in patients with macular edema has not been evaluated. Macular edema over an extended period of time (i.e. 6 months) can lead to permanent visual loss. Consider discontinuing ZEPOSIA if macular edema develops. The risk of recurrence after rechallenge has not been evaluated.
Patients with a history of uveitis and patients with a history of diabetes mellitus are at increased risk of macular edema during ZEPOSIA therapy.
Cutaneous Malignancies: The risk of cutaneous malignancies (including basal cell carcinoma, squamous cell carcinoma, and melanoma) is increased in patients treated with S1P receptor modulators. Skin examinations are recommended prior to or shortly after the start of treatment and periodically thereafter for all patients, particularly those with risk factors for skin cancer. Providers and patients are advised to monitor for suspicious skin lesions. If a suspicious skin lesion is observed, it should be promptly evaluated. As usual for patients with increased risk for skin cancer, exposure to sunlight and ultraviolet light should be limited by wearing protective clothing and using sunscreen with a high protection factor. Concomitant phototherapy with UV-B radiation or PUV-A photochemotherapy is not recommended in patients taking ZEPOSIA.
Posterior Reversible Encephalopathy Syndrome (PRES): Rare cases of PRES have been reported in patients receiving a S1P receptor modulator. If a ZEPOSIA-treated patient develops unexpected neurological or psychiatric symptoms or any symptom/sign suggestive of an increase in intracranial pressure, a complete physical and neurological examination should be conducted. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, treatment with ZEPOSIA should be discontinued.
Unintended Additive Immunosuppressive Effects From Prior Immunosuppressive or Immune-Modulating Drugs: When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects while at the same time minimizing risk of disease reactivation.
Immune System Effects After Stopping ZEPOSIA: After discontinuing ZEPOSIA, the median time for lymphocyte counts to return to the normal range was 30 days, with approximately 80% to 90% of patients in the normal range within 3 months. Use of immunosuppressants within this period may lead to an additive effect on the immune system, therefore caution should be applied when initiating other drugs 4 weeks after the last dose of ZEPOSIA.
Most Common Adverse Reactions (≥4%): liver test increased, upper respiratory infection, and headache.
Use in Specific Populations: Hepatic Impairment: Dosage adjustment in patients with mild or moderate hepatic impairment (Child-Pugh class A or B) is required, and use of ZEPOSIA in patients with severe hepatic impairment (Child-Pugh class C) is not recommended.
For additional safety information, please see the full
Prescribing Information and
Medication Guide.
References:
- Khan N, Abbas A, Williamson A, Balart L. Prevalence of corticosteroids use and disease course after initial steroid exposure in ulcerative colitis. Dig Dis Sci. 2013;58(10):2963-2969.
- Burri E, Maillard MH, Schoepfer AM, et al. Treatment algorithm for mild and moderate-to-severe ulcerative colitis: an update. Digestion. 2020;101(suppl 1):2-15.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(suppl 3):s1-s106.
- Zeposia. Prescribing Information. Bristol-Myers Squibb Company; 2024.
- Efficacy and safety study of ozanimod in ulcerative colitis (Touchstone). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/record/NCT01647516. Updated May 19, 2021. Accessed May 11, 2022.
References:
- Zeposia. Prescribing Information. Bristol-Myers Squibb Company; 2024.
- Sandborn WJ, Feagan BG, D’Haens G, et al; True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385(14):1280-1291.
- Sandborn WJ, Feagan BG, D’Haens G, et al; True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis [supplementary appendix]. N Engl J Med. 2021;385(14):1280-1291.
- Danese S, Colombel JF, Ponich T, et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis. Oral Presentation at: ECCO 2022; February 16-19, 2022. Presentation DOP44.
- Data on File. BMS-REF-OZA-0031. Princeton, NJ; Bristol-Myers Squibb Company; 2022.
- Data on File. BMS-REF-OZA-0022. Princeton, NJ; Bristol-Myers Squibb Company; 2022.
- Wolf DC, Colombel JF, Ponich T, et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis. Poster presented at: Digestive Disease Week (DDW); San Diego, CA, and Virtual, May 21–24, 2022. Poster Tu1458.
- Long M, Cross R, Calkwood J, et al. Ozanimod first-dose cardiac effects in patients with moderately to severely active ulcerative colitis and relapsing multiple sclerosis. Poster presented at AIBD 2021; December 9–11, 2021.
References:
- Irving P, Long MD, Siegmund B, et al. Efficacy of ozanimod as an induction therapy in advanced therapy-naive ulcerative colitis patients suboptimally controlled on conventional treatments. Oral presentation at: United European Gastroenterology (UEG) Week 2023; Copenhagen, Denmark; October 14–17, 2023. Presentation OP133.
- Sandborn WJ, Feagan BG, D’Haens G, et al; True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis [supplementary appendix]. N Engl J Med. 2021;385(14):1280-1291.
- Siegmund B, Axelrad J, Pondel M, et al. Rapidity of ozanimod-induced symptomatic response and remission in patients with moderately to severely active ulcerative colitis: results from the induction period of True North. Oral presentation at: European Crohn’s and Colitis Organisation (ECCO) Virtual; February 16–19, 2022.
- Zeposia. Prescribing Information. Bristol-Myers Squibb Company; 2024.
- Data on File. BMS-REF-OZA-0066. Princeton, NJ: Bristol-Myers Squibb Company; 2023.
- Panaccione R, Afzali A, Hudesman D, et al. Extended therapy with ozanimod for delayed responders to ozanimod in moderately to severely active ulcerative colitis: data from the True North open-label extension study. Poster presented at: Digestive Disease Week (DDW); San Diego, CA, and Virtual; May 21–24, 2022. Poster Tu1450.
- Siegmund B, Irving P, D’Haens G, et al. Ozanimod as a maintenance therapy in advanced therapy-naive ulcerative colitis patients with moderate vs severe endoscopic disease. Oral presentation at: United European Gastroenterology (UEG) Week 2023; Copenhagen, Denmark; October 14–17, 2023. Presentation OP135.
- Danese S, Colombel JF, Ponich T, et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis. Oral presentation at: ECCO 2022: February 16–19, 2022. Presentation DOP44.
- Danese S, Abreu MT, Wolf DC, et al. Efficacy and safety of 3 years of continuous ozanimod treatment: an interim analysis of the True North open-label extension study. Oral presentation at: European Crohn’s and Colitis Organisation (ECCO) 2023; Copenhagen, Denmark; March 1–4, 2023. Presentation DOP37.
- Sandborn WJ, Feagan BG, Hanauer S, et al. Long-term efficacy and safety of ozanimod in moderately to severely active ulcerative colitis: results from the open-label extension of the randomized, phase 2 TOUCHSTONE study.J Crohns Colitis. 2021;15(7):1120-1129.
- Panaccione R, Danese S, Wolf DC, et al. Long-term safety of 3 years of ozanimod in moderately to severely active ulcerative colitis: an interim analysis of the True North open-label extension. Poster presented at: European Crohn’s and Colitis Organisation (ECCO) 2023; Copenhagen, Denmark; March 1–4, 2023. Poster P405.
- Data on File. BMS_ZEP008535. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
- Wolf DC, Colombel JF, Ponich T, et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis. Poster presented at: Digestive Disease Week (DDW); San Diego, CA, and Virtual; May 21–24, 2022. Poster Tu1458.
- Danese S, Panaccione R, Abreu MT, et al. Efficacy and safety of approximately 3 years of continuous ozanimod in moderately to severely active ulcerative colitis: interim analysis of the True North open-label extension. J Crohns Colitis. Published online August 31, 2023. doi:10.1093/ecco-jcc/jjad146
References:
- Zeposia. Prescribing Information. Bristol-Myers Squibb Company; 2024.
- Irving P, Long MD, Siegmund B, et al. Efficacy of ozanimod as an induction therapy in advanced therapy–naive ulcerative colitis patients suboptimally controlled on conventional treatments. Oral presentation at: United European Gastroenterology (UEG) Week 2023; Copenhagen, Denmark; October 14–17, 2023. Presentation OP133.
References:
- Efficacy and safety study of ozanimod in ulcerative colitis (Touchstone). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT01647516. Updated May 19, 2021. Accessed July 26, 2023.
- Zeposia. Prescribing Information. Bristol-Myers Squibb Company; 2024.
- Study of ozanimod (RPC1063) in relapsing multiple sclerosis (MS) (SUNBEAM). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT02294058. Updated November 25, 2020. Accessed July 26, 2023.
- Efficacy and safety study of ozanimod in relapsing multiple sclerosis (RADIANCE). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT02047734. Updated February 11, 2021. Accessed July 26, 2023.
- Efficacy and safety study of ozanimod (RPC1063) in relapsing multiple sclerosis patients (RADIANCE). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT01628393. Updated November 2, 2021. Accessed September 6, 2023.
- Sandborn WJ, Feagan BG, D’Haens G, et al; True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385(14):1280-1291.
- Safety and efficacy trial of RPC1063 for moderate to severe ulcerative colitis. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT02435992. Updated September 1, 2021. Accessed July 26, 2023.
- An extension study of RPC1063 as therapy for moderate to severe ulcerative colitis. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT02531126. Updated September 18, 2023. Accessed September 25, 2023.
- A multi-site, open-label extension trial of oral RPC1063 in relapsing multiple sclerosis. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT02576717. Updated January 30, 2024. Accessed February 6, 2024.
- Nakase H, Fujii T, Hisamatsu T, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis: a randomized, double-blind, placebo-controlled study in Japan (J-True North). Poster presented at: Digestive Disease Week (DDW) 2024; Washington, DC; May 18-21, 2024. Poster Su1795.
- Study describing cognitive processing speed changes in relapsing multiple sclerosis subjects treated with ozanimod (RPC-1063) (ENLIGHTEN). Clinicaltrials.gov. https://clinicaltrials.gov/study/NCT0414035. Updated November 15, 2023. Accessed June 18, 2024.
- Data on File. BMS-REF-OZA-0076. Princeton, NJ: Bristol-Myers Squibb Company; 2024.
- Data on File. BMS-REF-OZA-0077. Princeton, NJ: Bristol-Myers Squibb Company; 2024.
- Selmaj KW, Steinman L, Comi G, et al. Long-term safety and efficacy of ozanimod in relapsing multiple sclerosis: final analysis of the DAYBREAK open-label extension study. Poster presented at: ACTRIMS 2024 Forum; February 29–March 2, 2024; West Palm Beach, FL. Poster P090.
- Press Release. U.S. Food and Drug Administration approves Bristol Myers Squibb’s ZEPOSIA® (ozanimod), a new oral treatment for relapsing forms of multiple sclerosis. Princeton, NJ: Bristol-Myers Squibb Company; 2020.
- Press Release. U.S. Food and Drug Administration approves Bristol Myers Squibb’s ZEPOSIA® (ozanimod), an oral treatment for adults with moderately to severely active ulcerative colitis. Princeton, NJ: Bristol-Myers Squibb Company; 2021.
- Data on File. BMS-REF-0079. Princeton, NJ: Bristol-Myers Squibb Company; 2024.
- Siegel CA, Danese S, Rubin DT, et al. Safety of long-term ozanimod treatment for up to 4 years in patients with moderately to severely active ulcerative colitis: an interim analysis of the True North open-label extension. Oral presentation at: European Crohn's and Colitis Organisation (ECCO) 2024; Stockholm, Sweden; February 21–24, 2024.
References:
- Sandborn WJ, Feagan BG, D’Haens G, et al; True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385(14):1280-1291.
- Zeposia. Prescribing Information. Bristol-Myers Squibb Company; 2024.
- Panaccione R, Danese S, Wolf DC, et al. Long-term safety of 3 years of ozanimod in moderately to severely active ulcerative colitis: an interim analysis of the True North open-label extension. Poster presented at: European Crohn’s and Colitis Organisation (ECCO) 2023; Copenhagen, Denmark; March 1–4, 2023. Poster P405.
- Efficacy and safety study of ozanimod in ulcerative colitis (Touchstone). ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT01647516. Updated May 19, 2021. Accessed July 26, 2023.
- Long M, Cross RK, Lichtenstein GR, et al. Long-term cardiac safety of ozanimod in phase 3 clinical program of ulcerative colitis and relapsing multiple sclerosis. Presented at: Digestive Disease Week (DDW); San Diego, CA, and Virtual; May 21–24, 2022. Presentation 15.
- Rieder F, Wolf DC, Charles L, Kollengode K, Patel A, Ghosh S. Incidence of infections in patients with moderately to severely active ulcerative colitis treated with ozanimod and relationship to significant lymphopenia: results from a pooled safety analysis. Poster presented at: DDW 2021 Virtual Digestive Disease Week; May 21–23, 2021.
- Danese S, Feagan BG, Wolf DC. Ozanimod as maintenance therapy in patients with moderate-to-severe ulcerative colitis: results from the phase 3, randomized, double-blind, placebo-controlled True North study. Paper presented at: United European Gastroenterology Week Virtual 2020; October 10–14, 2020. Presentation LB10.
- D’Haens G, Colombel JF, Lichtenstein GR, et al. Safety of ozanimod in patients with moderately to severely active ulcerative colitis over time: pooled analysis from phase 2, phase 3, and open-label extension trials. Oral presentation at: DDW 2021 Virtual Digestive Disease Week; May 21–23, 2021.
- Sandborn WJ, Feagan BG, Hanauer S, et al. Long-term efficacy and safety of ozanimod in moderately to severely active ulcerative colitis: results from the open-label extension of the randomized, phase 2 TOUCHSTONE study. J Crohns Colitis. 2021;15(7):1120-1129.
- Siegel CA, Danese S, Rubin DT, et al. Safety of long-term ozanimod treatment for up to 4 years in patients with moderately to severely active ulcerative colitis: an interim analysis of the True North open-label extension. Oral presentation at: European Crohn's and Colitis Organisation (ECCO) 2024; Stockholm, Sweden; February 21–24, 2024.
- Data on File. BMS-REF-OZA-0066. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
- Wolf DC, Colombel JF, Ponich T, et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis. Poster presented at: Digestive Disease Week (DDW); San Diego, CA, and Virtual; May 21–24, 2022. Poster Tu1458.
References:
- Zeposia. Prescribing Information. Bristol-Myers Squibb Company; 2024.
- Sandborn WJ, Vermeire S, Peyrin-Biroulet L, et al. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies [published correction appears in Lancet. 2023 Mar 25;401(10381):1000]. Lancet. 2023;401(10383):1159-1171.
- McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions [published correction appears in Lancet. 2021 Sep 25;398(10306):1132]. Lancet. 2021;398(10306):1184-1194.
- Roggeri A, Schepers M, Tiane A, et al. Sphingosine-1-phosphate receptor modulators and oligodendroglial cells: beyond immunomodulation. Int J Mol Sci. 2020;21(20):7537.
- Pérez-Jeldres T, Tyler CJ, Boyer JD, et al. Cell trafficking interference in inflammatory bowel disease. Inflamm Bowel Dis. 2019;2(25):270-282.
- Al-Bawardy B, Shivashankar R, Proctor DD. Novel and emerging therapies for inflammatory bowel disease. Front Pharmacol. 2021;12:651415.
- Nikolakis D, de Voogd FAE, Pruijit MJ, et al. The role of the lymphatic system in the pathogenesis and treatment of inflammatory bowel disease. Int J Mol Sci. 2022;23(3):1854.
- Harris S, Tran JQ, Southworth H, Spencer CM, Cree BAC, Zamvil SS. Effect of the sphingosine-1-phosphate receptor modulator ozanimod on leukocyte subtypes in relapsing MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e839.
- Jaigirdar SA, Benson RA, Elmesmari A, et al. Sphingosine 1-phosphate promotes the persistence of activated CD4 T cells in inflamed sites. Front Immunol. 2017;8:1627.
- Scott FL, Clemons B, Brooks J, et al. Ozanimod (RPC1063) is a potent sphingosine 1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173:1778-1792.
- Kayal M, Shah S. Ulcerative colitis: current and emerging treatment strategies. J Clin Med. 2019;9(1):94.
- Olivera P, Danese S, Peyrin-Biroulet L. Next generation of small molecules in inflammatory bowel disease. Gut. 2017;66(2):199-209.
- Lucaciu LA, Seicean R, Seicean A. Small molecule drugs in the treatment of inflammatory bowel diseases: which one, when and why? – a systematic review. Eur J Gastroenterol Hepatol. 2020;32(6):669-677.
Reference:
- Zeposia. Prescribing Information. Bristol-Myers Squibb Company; 2024.