The primary endpoint for the TRUE NORTH clinical trial was clinical remission at Week 10 and Week 52.
Patients experienced reliefc from symptoms as early as Week 21-3
A decrease in both RBS and
SFS was observed in
patients taking ZEPOSIA as
early as Week 22,4
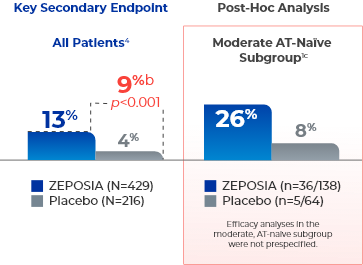
Rapid clinical
response (key secondary endpoint)
at Week 104a
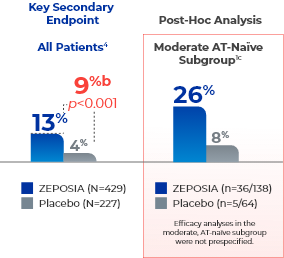
Long-term clinical
remission data observed
up to ~4 years5
- aClinical response is defined as a reduction from baseline in the 3-component Mayo score of ≥2 points and ≥35%, and a reduction from baseline in the RBS of ≥1 point or an absolute RBS of 0 or 1. Significantly higher clinical response rates vs placebo in the pivotal trial: 48% (205/429) vs 26% (56/216) at Week 10 (p<0.0001); 60% (138/230) vs 41% (93/227) at Week 52 (p<0.0001).4
- bClinical remission is defined as RBS=0, SFS=0 or 1 (and a decrease of ≥1 point from baseline SFS), and endoscopy subscore=0 or 1 without friability. Significantly higher clinical remission rates vs placebo in the pivotal trial: 18% (79/429) vs 6% (13/216) at Week 10 (p<0.0001); 37% (85/230) vs 19% (42/227) at Week 52 (p<0.0001).4
- cSymptomatic clinical response was defined as a decrease from baseline in the combined 6-point SFS + RBS by ≥1 point and ≥30%, and a decrease of ≥1 point in RBS or an absolute RBS of ≤1 point.3
RBS=rectal bleeding subscore; SFS=stool frequency subscore.
Week 10 Induction Data
Post-Hoc Analysis:
Symptomatic Response
Clinical
Response
Clinical
Remission
Endoscopic
Improvement
EHMI
Post-hoc analysis: patients experienced reliefa from UC symptoms as early as 1 week after completing the 7-day dose titration1-3
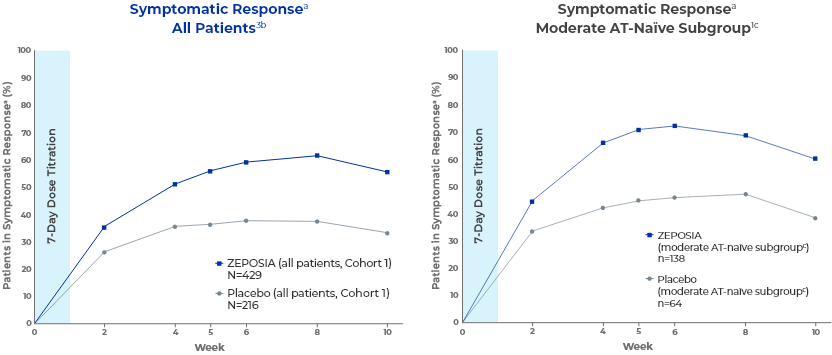
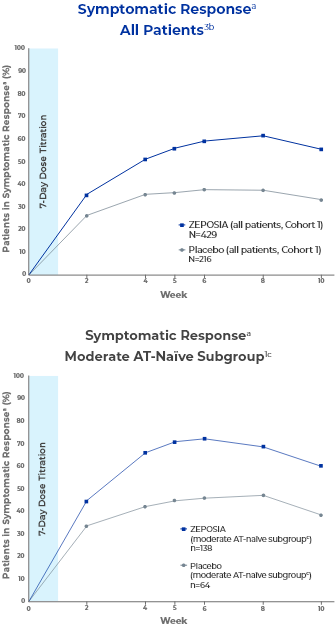
A decrease in both RBS and SFS was observed in patients taking ZEPOSIA as early as Week 2.2,4
These post-hoc analyses were not prespecified.1
- aSymptomatic clinical response is defined as a decrease from baseline in the combined 6-point SFS + RBS by ≥1 point and ≥30%, and a decrease of ≥1 point in RBS or an absolute RBS of ≤1 point.1,3
- bData are based on the nonresponder imputation.3
- cThe moderate AT-naïve subgroup included patients with a Mayo endoscopy subscore of 2.1
AT=advanced therapy; EHMI=endoscopic-histologic mucosal improvement; OLE=open-label extension; RBS=rectal bleeding subscore; SFS=stool frequency subscore; TNFi=tumor necrosis factor inhibitor; UC=ulcerative colitis.
Secondary Endpoint
ZEPOSIA delivers rapid clinical response at Week 104a
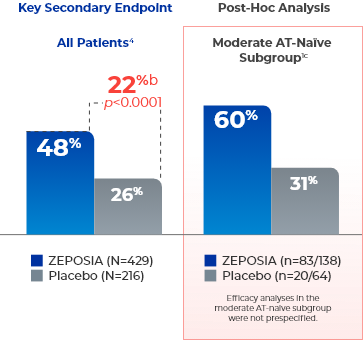
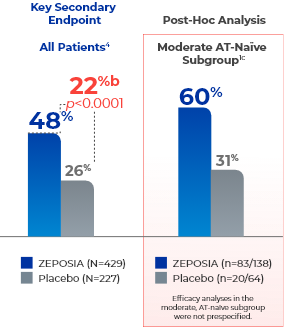
- aClinical response is defined as a reduction from baseline in the 3-component Mayo score of ≥2 points and ≥35%, and a reduction from baseline in the RBS of ≥1 point or an absolute RBS of 0 or 1.1,4
- bTreatment difference (adjusted for stratification factors of prior TNFi exposure and corticosteroid use at baseline).4
- cThe moderate AT-naïve subgroup included patients with a Mayo endoscopy subscore of 2.1
Post-hoc analysis:
49% of nonrespondersd who entered the open-label extension at Week 10 after the
induction period observed symptomatic clinical response by Week 20e (open-label extension
Week 10)6
- Symptomatic clinical response is defined as a reduction from baseline in the partial Mayo score of ≥1 point and ≥30% and a ≥1-point decrease in RBS or absolute RBS ≤1.1,3
- Efficacy analyses of symptomatic response were not prespecified.1
- dNonresponders were patients who did not achieve clinical response (defined as a reduction from baseline in the 3-component Mayo score of ≥2 points and ≥35%, and a reduction from baseline in the RBS of ≥1 point or an absolute RBS of 0 or 1) on ZEPOSIA at Week 10 of induction and entered the OLE.4,6
- eData are based on the nonresponder imputation.6
AT=advanced therapy; EHMI=endoscopic-histologic mucosal improvement; OLE=open-label extension; RBS=rectal bleeding subscore; SFS=stool frequency subscore; TNFi=tumor necrosis factor inhibitor; UC=ulcerative colitis.
Primary Endpoint
ZEPOSIA delivers rapid clinical remission at Week 104a
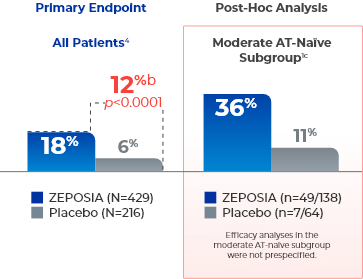
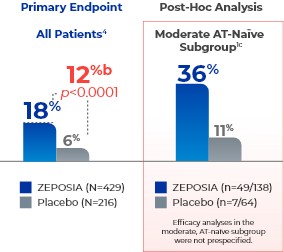
- aClinical remission is defined as RBS=0, SFS=0 or 1 (and a decrease of ≥1 point from baseline SFS), and endoscopy subscore=0 or 1 without friability.1,4
- bTreatment difference (adjusted for stratification factors of prior TNFi exposure and corticosteroid use at baseline).4
- cThe moderate AT-naïve subgroup included patients with a Mayo endoscopy subscore of 2.1
AT=advanced therapy; EHMI=endoscopic-histologic mucosal improvement; OLE=open-label extension; RBS=rectal bleeding subscore; SFS=stool frequency subscore; TNFi=tumor necrosis factor inhibitor; UC=ulcerative colitis.
Secondary Endpoint
Patients taking ZEPOSIA saw significant endoscopic improvement at Week 104a
- aEndoscopic improvement is defined as a Mayo endoscopy subscore of 0 or 1 without friability.1,4
- bTreatment difference (adjusted for stratification factors of prior TNFi exposure and corticosteroid use at baseline).4
- cThe moderate AT-naïve subgroup included patients with a Mayo endoscopy subscore of 2.1
AT=advanced therapy; EHMI=endoscopic-histologic mucosal improvement; OLE=open-label extension; RBS=rectal bleeding subscore; SFS=stool frequency subscore; TNFi=tumor necrosis factor inhibitor; UC=ulcerative colitis.
- The relationship of EHMI, as defined in UC Study 1 at Week 10, to disease progression and long-term outcomes was not evaluated.4
- aEHMI is defined as both a Mayo endoscopy subscore of 0 or 1 without friability and histologic improvement of colonic tissue (defined as no neutrophils in the epithelial crypts or lamina propria and no increase in eosinophils, no crypt destruction, and no erosions, ulcerations, or granulation tissue, ie, Geboes <2.0).1,4
- bTreatment difference (adjusted for stratification factors of prior TNFi exposure and corticosteroid use at baseline).4
- cThe moderate AT-naïve subgroup included patients with a Mayo endoscopy subscore of 2.1
AT=advanced therapy; EHMI=endoscopic-histologic mucosal improvement; OLE=open-label extension; RBS=rectal bleeding subscore; SFS=stool frequency subscore; TNFi=tumor necrosis factor inhibitor; UC=ulcerative colitis.
Week 52 Maintenance Data
Clinical
Response
Clinical
Remission
CS-Free
Remission
Endoscopic
Improvement
EHMI
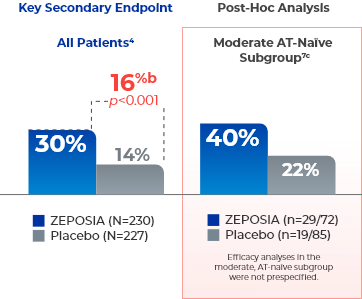
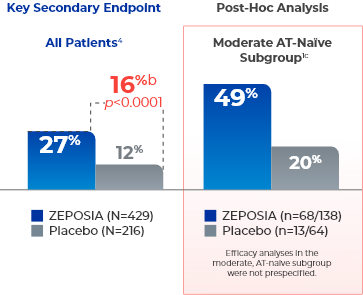
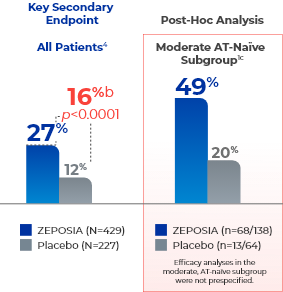
Secondary Endpoint
Increased rates of clinical response seen at Week 524a
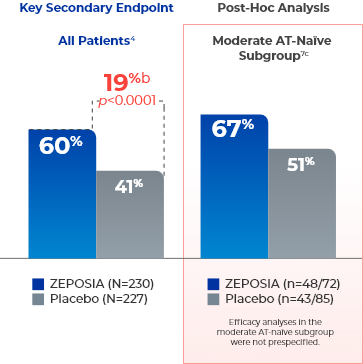
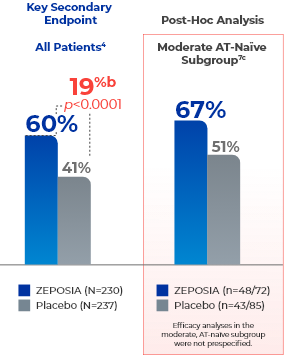
- aClinical response is defined as a reduction from baseline in the 3-component Mayo score of ≥2 points and ≥35%, and a reduction from baseline in the RBS of ≥1 point or an absolute RBS of 0 or 1.4,7
- bTreatment difference (adjusted for stratification factors of clinical remission and concomitant corticosteroid use at Week 10).4
- cThe moderate AT-naïve subgroup included patients with a Mayo endoscopy subscore of 2.7
AT=advanced therapy; CS=corticosteroid; EHMI=endoscopic-histologic mucosal improvement; RBS=rectal bleeding subscore; SFS=stool frequency subscore; TNFi=tumor necrosis factor inhibitor; UC=ulcerative colitis.
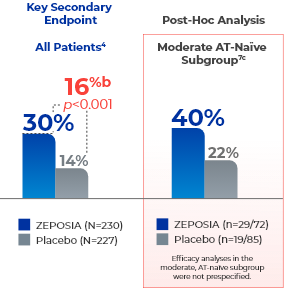
Primary Endpoint
ZEPOSIA demonstrates sustained clinical remission at Week 524a
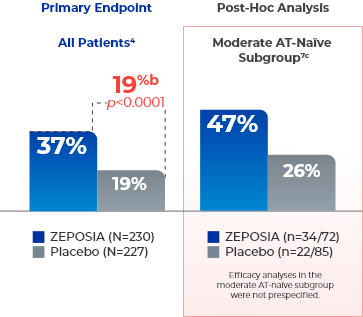
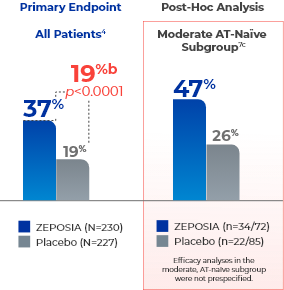
- aClinical remission is defined as RBS=0, SFS=0 or 1 (and a decrease of ≥1 point from baseline SFS), and endoscopy subscore=0 or 1 without friability.4,7
- bTreatment difference (adjusted for stratification factors of clinical remission and concomitant corticosteroid use at Week 10).4
- cThe moderate AT-naïve subgroup included patients with a Mayo endoscopy subscore of 2.7
AT=advanced therapy; CS=corticosteroid; EHMI=endoscopic-histologic mucosal improvement; RBS=rectal bleeding subscore; SFS=stool frequency subscore; TNFi=tumor necrosis factor inhibitor; UC=ulcerative colitis.
Secondary Endpoint
ZEPOSIA can help patients achieve remission without the use of corticosteroids4a
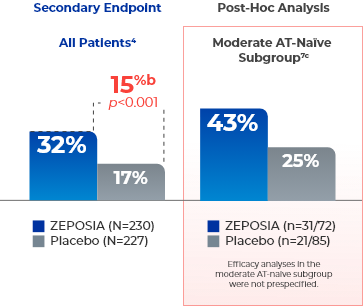
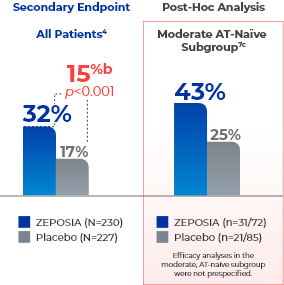
- aCS-free remission is defined as clinical remission at Week 52 while off corticosteroids for ≥12 weeks.4,7
- bTreatment difference (adjusted for stratification factors of clinical remission and concomitant corticosteroid use at Week 10).4
- cThe moderate AT-naïve subgroup included patients with a Mayo endoscopy subscore of 2.7
AT=advanced therapy; CS=corticosteroid; EHMI=endoscopic-histologic mucosal improvement; RBS=rectal bleeding subscore; SFS=stool frequency subscore; TNFi=tumor necrosis factor inhibitor; UC=ulcerative colitis.
Secondary Endpoint
Significant endoscopic improvement observed at Week 524a
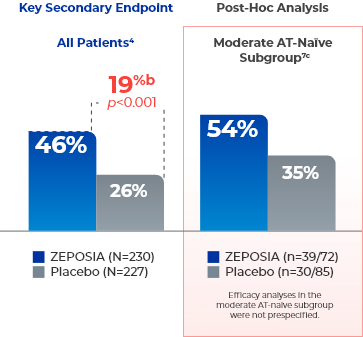
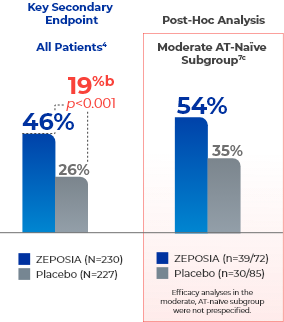
- aEndoscopic improvement is defined as a Mayo endoscopy subscore of 0 or 1 without friability.4,7
- bTreatment difference (adjusted for stratification factors of clinical remission and concomitant corticosteroid use at Week 10).4
- cThe moderate AT-naïve subgroup included patients with a Mayo endoscopy subscore of 2.7
AT=advanced therapy; CS=corticosteroid; EHMI=endoscopic-histologic mucosal improvement; RBS=rectal bleeding subscore; SFS=stool frequency subscore; TNFi=tumor necrosis factor inhibitor; UC=ulcerative colitis.
- The relationship of EHMI, as defined in UC Study 2 at Week 52, to disease progression and long-term outcomes was not evaluated.4
- aEHMI is defined as both a Mayo endoscopy subscore of 0 or 1 without friability and histologic improvement of colonic tissue (defined as no neutrophils in the epithelial crypts or lamina propria and no increase in eosinophils, no crypt destruction, and no erosions, ulcerations, or granulation tissue, ie, Geboes <2.0).4,7
- bTreatment difference (adjusted for stratification factors of clinical remission and concomitant corticosteroid use at Week 10).4
- cThe moderate AT-naïve subgroup included patients with a Mayo endoscopy subscore of 2.7
AT=advanced therapy; CS=corticosteroid; EHMI=endoscopic-histologic mucosal improvement; RBS=rectal bleeding subscore; SFS=stool frequency subscore; TNFi=tumor necrosis factor inhibitor; UC=ulcerative colitis.
Long-Term Clinical Data Observed up to ~4 Years5
Clinical
Remission
Clinical
Response
CS-Free
Remission
Clinical remissiona data up to 194 weeks in the open-label extension trial5
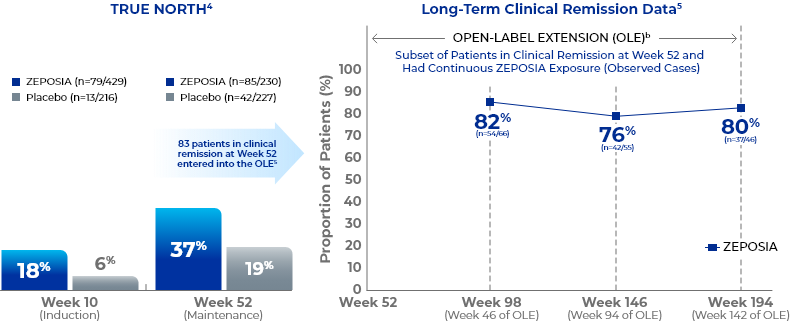
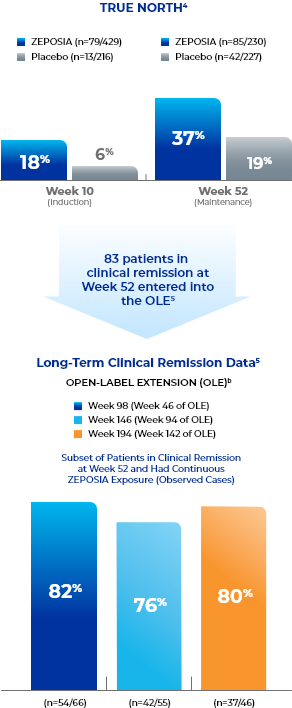
- Study Design for OLE
- The TRUE NORTH OLE is an ongoing trial that enrolled participants who were nonresponders at the end of induction, experienced disease relapse during maintenance, or completed maintenance treatment in the phase 3 TRUE NORTH study or remained at study closure and received once-daily oral ZEPOSIA 0.92 mg in the phase 2 TOUCHSTONE OLE. A total of 823 patients from TRUE NORTH entered the TRUE NORTH OLE.8-10
- Endpoints were evaluated at Weeks 46, 94, and 142 of the OLE for all patients who entered the OLE from the TRUE NORTH parent study and for a subset of patients in clinical remission or clinical response at Week 52 and had continuous ZEPOSIA exposure. Endpoints include clinical remission, clinical response, endoscopic improvement, and CS-free remission. Safety was evaluated for all 823 patients who entered the OLE from the TRUE NORTH parent study.8,9,11-13
- These analyses were not prespecified and represent a subgroup of all patients from TRUE NORTH who entered the OLE.13
- aClinical remission (3-component Mayo score) is defined as RBS=0, SFS ≤1 (and a decrease of ≥1 point from the baseline SFS), and endoscopy subscore ≤1.5
- bThe OLE did not include a placebo comparator arm.5,14
CS=corticosteroid; ITT=intent-to-treat; NRI=nonresponder imputation; OC=observed case; RBS=rectal bleeding subscore; SFS=stool frequency subscore.
Clinical responsea data up to 194 weeks in the open-label extension trial5
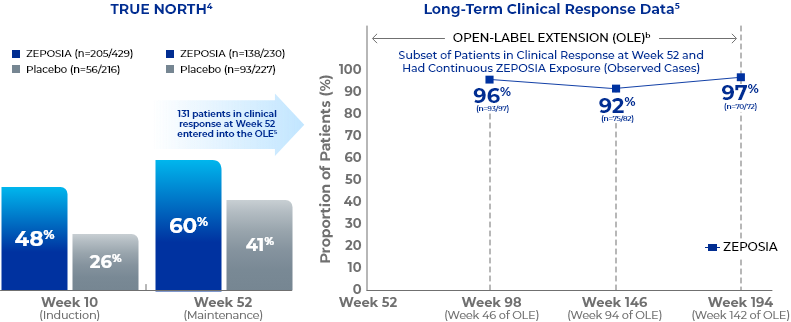
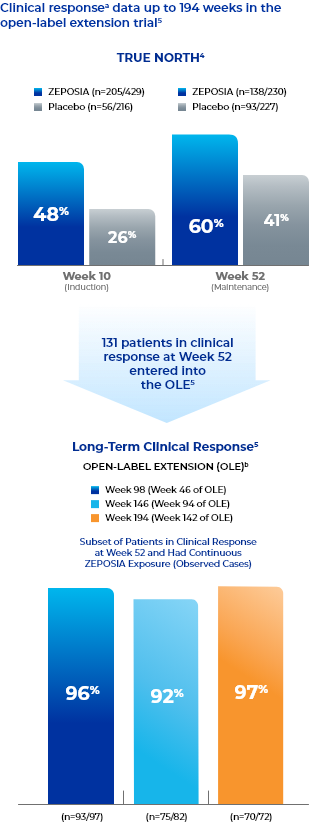
- Study Design for OLE
- The TRUE NORTH OLE is an ongoing trial that enrolled participants who were nonresponders at the end of induction, experienced disease relapse during maintenance, or completed maintenance treatment in the phase 3 TRUE NORTH study or remained at study closure and received once-daily oral ZEPOSIA 0.92 mg in the phase 2 TOUCHSTONE OLE. A total of 823 patients from TRUE NORTH entered the TRUE NORTH OLE.8-10
- Endpoints were evaluated at Weeks 46, 94, and 142 of the OLE for all patients who entered the OLE from the TRUE NORTH parent study and for a subset of patients in clinical remission or clinical response at Week 52 and had continuous ZEPOSIA exposure. Endpoints include clinical remission, clinical response, endoscopic improvement, and CS-free remission. Safety was evaluated for all 823 patients who entered the OLE from the TRUE NORTH parent study.8,9,11-13
- These analyses were not prespecified and represent a subgroup of all patients from TRUE NORTH who entered the OLE.13
- aClinical response is defined as a reduction from baseline in the 3-component Mayo score of ≥2 points and ≥35%, and a reduction from baseline in the RBS of ≥1 point or an absolute RBS of 0 or 1.5
- bThe OLE did not include a placebo comparator arm.5,14
CS=corticosteroid; ITT=intent-to-treat; NRI=nonresponder imputation; OC=observed case; RBS=rectal bleeding subscore; SFS=stool frequency subscore.
CS-free remissiona data up to 194 weeks in the open-label extension trial5
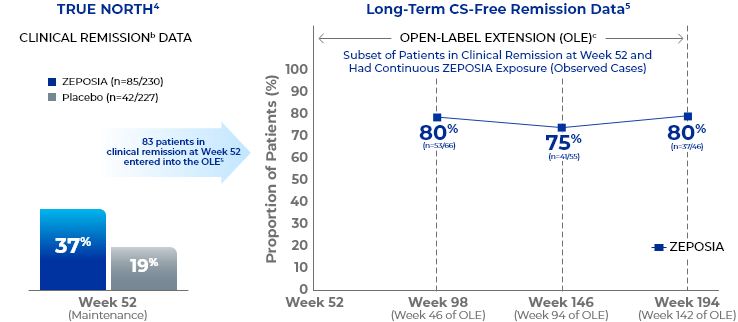
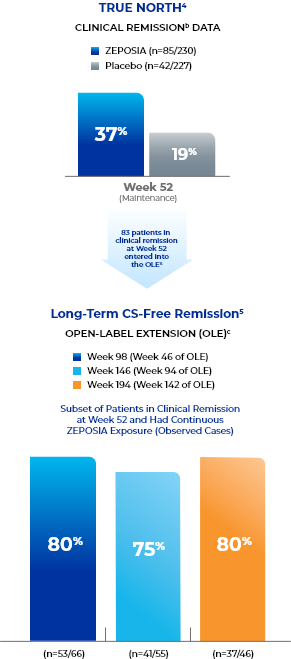
- Study Design for OLE
- The TRUE NORTH OLE is an ongoing trial that enrolled participants who were nonresponders at the end of induction, experienced disease relapse during maintenance, or completed maintenance treatment in the phase 3 TRUE NORTH study or remained at study closure and received once-daily oral ZEPOSIA 0.92 mg in the phase 2 TOUCHSTONE OLE. A total of 823 patients from TRUE NORTH entered the TRUE NORTH OLE.8-10
- Endpoints were evaluated at Weeks 46, 94, and 142 of the OLE for all patients who entered the OLE from the TRUE NORTH parent study and for a subset of patients in clinical remission or clinical response at Week 52 and had continuous ZEPOSIA exposure. Endpoints include clinical remission, clinical response, endoscopic improvement, and CS-free remission. Safety was evaluated for all 823 patients who entered the OLE from the TRUE NORTH parent study.8,9,11-13
- These analyses were not prespecified and represent a subgroup of all patients from TRUE NORTH who entered the OLE.13
- aCS-free remission is defined as clinical remission at Week 52 while off corticosteroids for ≥12 weeks.5
- bClinical remission is defined as RBS=0, SFS=0 or 1 (and a decrease of ≥1 point from baseline SFS), and endoscopy subscore=0 or 1 without friability.4
- cThe OLE did not include a placebo comparator arm.5,14
CS=corticosteroid; ITT=intent-to-treat; NRI=nonresponder imputation; OC=observed case; RBS=rectal bleeding subscore; SFS=stool frequency subscore.

This website is best viewed
using the horizontal display on
your tablet device.

This website is best viewed
using the vertical display on
your mobile device.