
ZEPOSIA 360 and Resources for Download
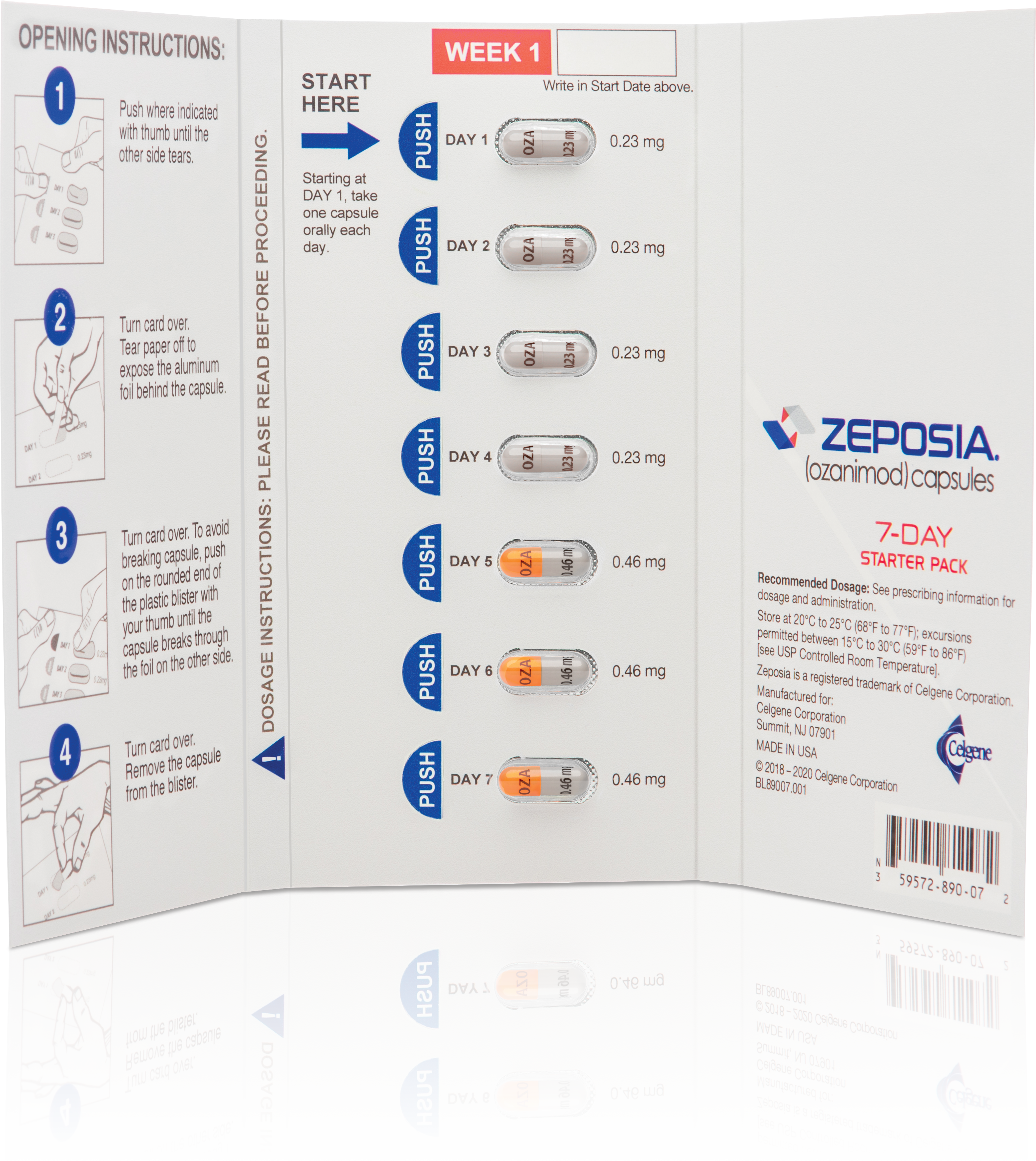
Eligible patients enrolled in ZEPOSIA 360 Support™, who have been cleared to begin treatment, and who have not received a sample from their provider, will be provided a starter kit that includes a 7-day starter pack and a 30-day supply of ZEPOSIA.

ZEPOSIA 360 Support™ Helps Support Your Patients Every Step of the Way
Get your patients started on ZEPOSIA

BMS Esign enables your patients to electronically sign through www.BMSesign.com
BMS eSign
Did your patient forget to sign the ZEPOSIA Start Form? Send them a link to the form below to sign electronically via CoverMyMeds.
Get the resources you and your patients need
From helpful tools and support to get them started on ZEPOSIA to additional help for your office.
ZEPOSIA 360 Support™*

ZEPOSIA Starter Kit
New eligible patients enrolled in ZEPOSIA 360 Support™ may receive a free Starter Kit that includes a 7-day starter pack and a 21-dose supply of ZEPOSIA if they are cleared to begin treatment and have not received a sample

Access Support
The
 portal serves as a central location to manage and track your patients' access to ZEPOSIA. Access support through CoverMyMeds includes:
portal serves as a central location to manage and track your patients' access to ZEPOSIA. Access support through CoverMyMeds includes:
 portal serves as a central location to manage and track your patients' access to ZEPOSIA. Access support through CoverMyMeds includes:
portal serves as a central location to manage and track your patients' access to ZEPOSIA. Access support through CoverMyMeds includes: - Prior authorization and appeals support
- Digital start form for enrollment online
- Benefits verification with electronic tracking of your patients’ benefit status

Baseline Assessment Assistance
Eligible commercially insured patients may receive assessments in office or in their home

24-Month Bridge Program
A free supply of ZEPOSIA for up to 2 years for eligible commercially insured patients prescribed ZEPOSIA who are at risk of a delay or interruption in therapy

Co-Pay Assistance Program: May Pay as Little as $0
Eligible commercially insured patients may pay as little as $0 in out-of-pocket costs for ZEPOSIA and may also be reimbursed for out-of-pocket costs associated with baseline assessments
*Eligibility and Terms and Conditions apply. The accurate completion of reimbursement or coverage-related documentation is the responsibility of the healthcare provider and patient. Bristol Myers Squibb and its agents make no guarantees regarding reimbursement for any service or item.
Please click here to view full Terms and Conditions.
Resource Downloads
Enrollment Resources
Start Form
Enrolls patients in ZEPOSIA 360 Support™ and helps them get
started on treatment. Download the start form or get started online.
BMS eSign
Available for your patients who forget to sign the ZEPOSIA Start Form. BMS eSign
enables your patients to sign electronically via
CoverMyMeds.
Initiation and Support Brochure
Provides important information on how to get patients started on ZEPOSIA and an
overview of key benefits available through the ZEPOSIA 360 Support™ program.
Patient Brochure
Provides a streamlined version of the Patient Decision Kit, including a quick
overview of ZEPOSIA and ZEPOSIA 360 Support™.
Baseline Assessment Assistance Brochure
Outlines the process for in-home or in-office baseline assessment testing
assistance for eligible patients enrolled in ZEPOSIA 360 Support™.
ICD-10-CM Reference Flashcard
Provides important information on changes to the ICD-10-CM Codes for MS,
effective October 2025
Access
& Support
Authorization & Appeals Kit
Offers
information on potential coverage scenarios for ZEPOSIA and outlines services offered by ZEPOSIA
360 Support™.
Formulary Exception Letter
Helps you advocate for your patients, so
they can gain approval to start therapy
with ZEPOSIA.
they can gain approval to start therapy
with ZEPOSIA.
Letter for Medical Necessity for Patients Currently Receiving MS Treatment
Enables you to help your patients continue receiving therapy.
Letter for Medical Necessity for Patients Not Currently Receiving MS Treatment
Helps your patients not currently receiving any therapy to get started on
ZEPOSIA.
Letter of Appeal for Patients Currently Receiving MS Treatment
Enables you to advocate for patients who are currently receiving therapy, if
they receive an unfavorable coverage decision.
Letter of Appeal for Patients Not Currently Receiving MS Treatment
Enables you to advocate for patients who are not currently receiving therapy,
if they receive an unfavorable coverage decision.
Step Therapy Letter
Helps you advocate for your patients if step requirements apply, so they can
gain approval to start or continue prescribed therapy with ZEPOSIA.
Additional
Resources
Specialty Pharmacy Resource
Provides a quick overview of the benefits of using Specialty Pharmacies (SPs)
and a list of SPs ready to handle ZEPOSIA prescriptions.
Baseline Testing Completion Form
This form is used by the ZEPOSIA 360 Support™ clinical
partners to verify that a patient’s baseline tests have been reviewed by their prescriber and
that they are able to start therapy. This form needs to be submitted, either online or by fax.
If not, someone from ZEPOSIA 360 Support™ will contact you for verbal
confirmation of baseline test completion.
REQUEST A REP

This website is best viewed
using the horizontal display on
your tablet device.

This website is best viewed
using the vertical display on
your mobile device.




